Benzinger Lab
Projects
The Benzinger Lab has a number of ongoing projects that are recruiting at any given time. Below is an overview of what we are working on at the moment. To see our currently open clinical trials, visit ClinicalTrials.gov.
New Imaging Techniques
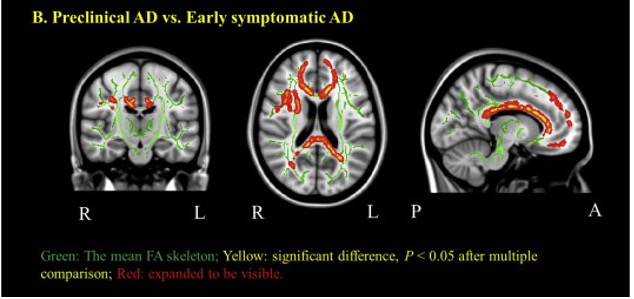
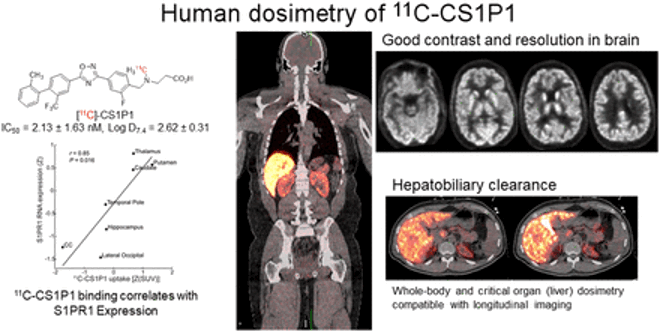
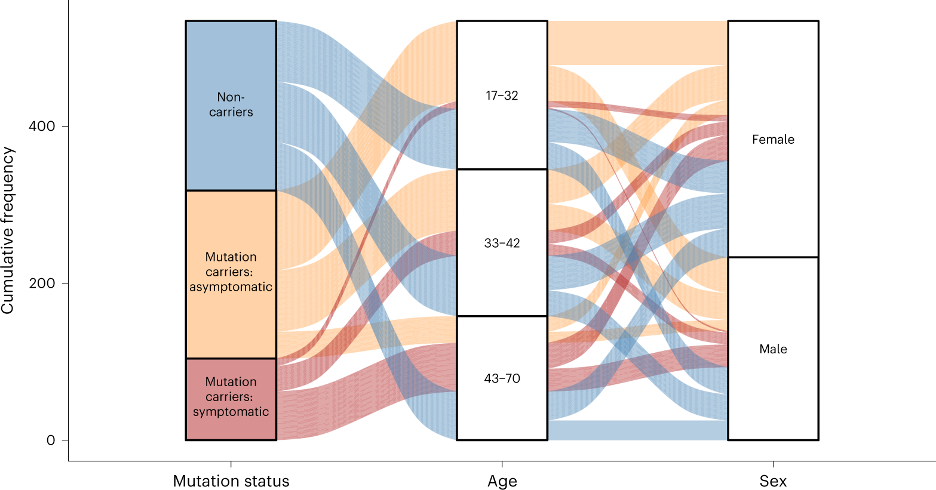
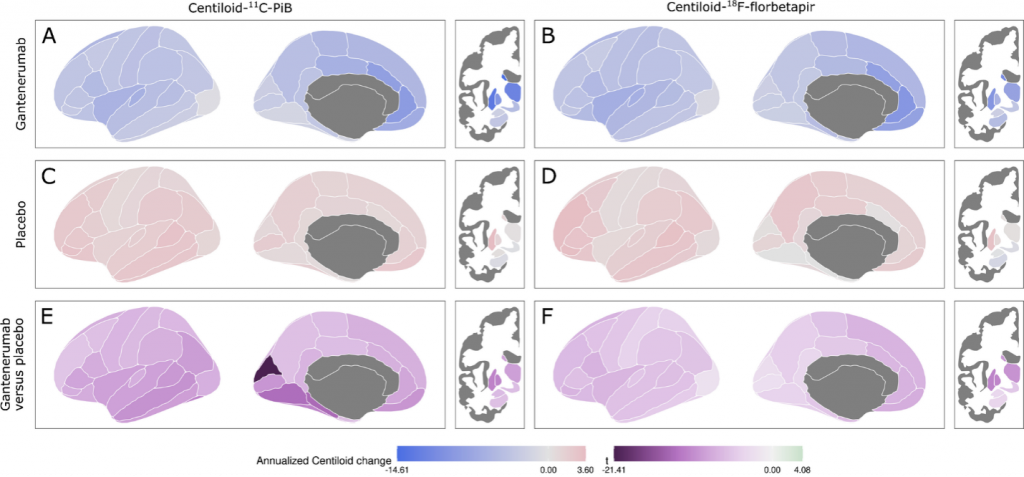
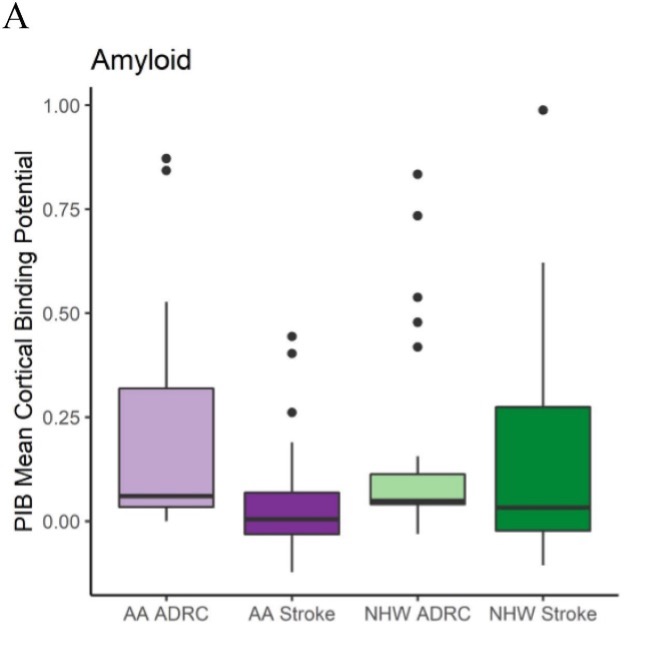
Alzheimer disease (AD) is preceded by at least a decade of clinically silent brain changes (termed “preclinical AD”) that ultimately result in declines in memory and thinking. Inflammation in the brain plays an important role neurodegenerative diseases, particularly in the transition from normal cognition to dementia, but we lack easily accessible tools to identify neuroinflammation. These projects use a novel brain MR imaging test, diffusion basis spectrum imaging (DBSI), and PET study with 11C-CS1P1 to examine inflammation during the stages of preclinical and clinical Alzheimer’s disease and also in multiple sclerosis.
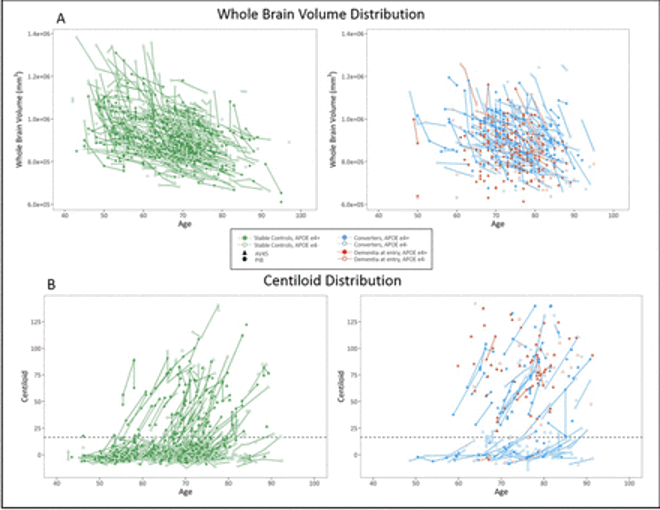
Knight Alzheimer Research Imaging (KARI) Program
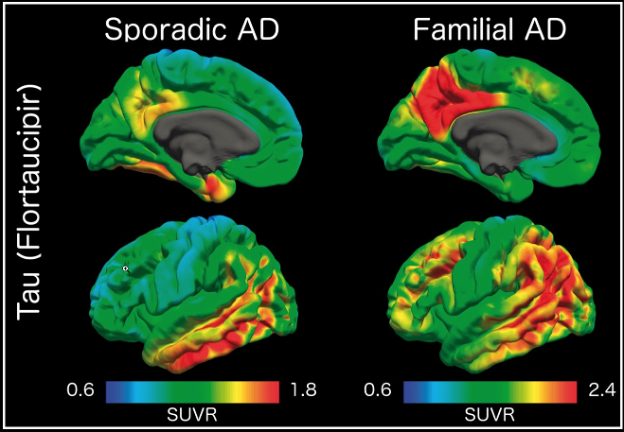
International Collaborations for Autosomal Dominant AD
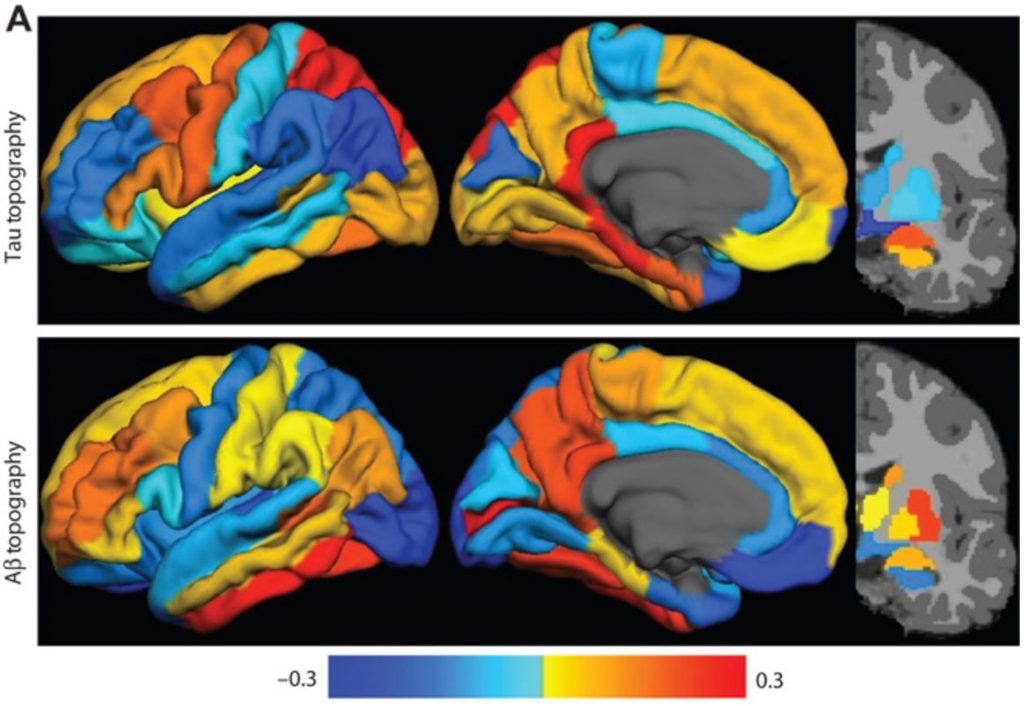
Dominantly Inherited Alzheimer Network (DIAN) Imaging Core Lab
An international study of families with autosomal dominant AD. Imaging includes MRI, amyloid PIB and AV45 PET, and tau T807 PET.
Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) Imaging Core Lab
An international study of families with autosomal dominant AD. Imaging includes MRI, amyloid PIB and AV45 PET, and tau T807 (AV-1451) PET.
Clinical Translational Research: Brain Tumors
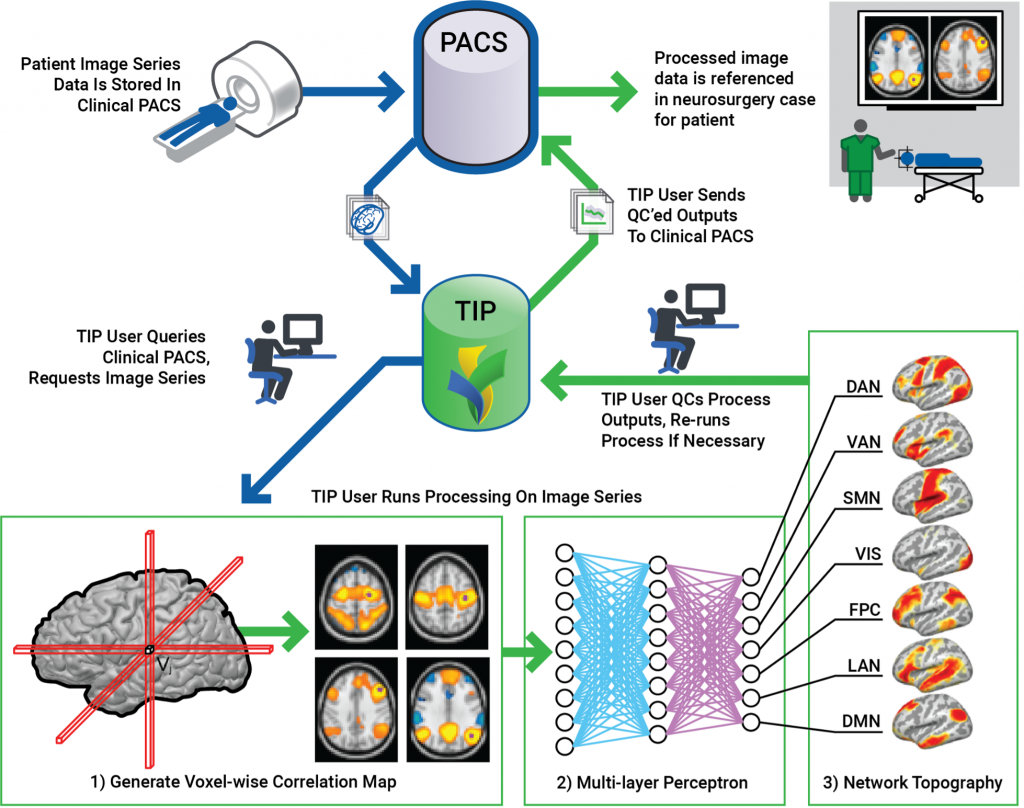
Neurosurgical Translational Interface Pipeline for Clinical Resting State Functional MRI
The goal of this project is to develop a clinical workflow for volumetric MRI and resting state functional MRI for preoperative planning and for evaluation of dementia.
Additional Sources of Funding
- Willman Scholar, The Foundation for Barnes-Jewish Hospital (supported Austin McCullough and Brian Gordon’s work in AD Research)
- Support for image acquisition – CCIR/ICTS Human Imaging Unit. NIH/NCATS UL1TR000448
- Translational Imaging in Radiopharmaceutical Sciences (TIRS) Research Program, a T32 postdoctoral training program
- CCIR additionally supported for Vision PET/CT: “This work was supported by NIH grant S10OD025214 for the Vision PET/CT Scanner housed in the Center for Clinical Imaging Research (CCIR), Mallinckrodt Institute of Radiology, Washington University in St. Louis.”
- Support for imaging informatics – XNAT “This study was supported in part by the Neuroimaging Informatics and Analysis Center (1P30NS098577) and R01 EB009352.”
Additional Conflicts of Interest
Tammie Benzinger, MD, PhD, has received investigator initiated research funding from the NIH, the Alzheimer’s Association, the Foundation at Barnes-Jewish Hospital, Siemens Healthineers and Avid Radiopharmaceuticals (a wholly-owned subsidiary of Eli Lilly and Company). She participates as a site investigator in clinical trials sponsored by Eli Lilly and Company, Biogen, Eisai, Jaansen, and Roche. She has served as a paid and unpaid consultant to Eisai, Siemens, Biogen, Janssen, and Bristol-Myers Squibb.