Woodard Lab
Projects
Receptor-Targeted Nanoparticle PET Tracer in Human Carotid Atherosclerosis
Goal
This project, ongoing since 2005, developed a novel radiotracer targeting the natriuretic peptide receptor-C (NPR-C) for the assessment of atherosclerosis. NPR-C is present in macrophages and vascular smooth muscle cells (VSMC) and is responsible for VSMC migration. It is present in atherosclerosis with features of vulnerability. We have translated this PET radiotracer into patients with demonstrated carotid atherosclerosis and are now in the process of pursuing its use across centers (Cedars-Sinai Medical Center) and assessing its uptake relative to patient outcomes. In patients who are imaged and undergo carotid endarterectomy surgery, we compare imaging features to plaque pathology, vulnerability and NPRC distribution within macrophage subtypes to facilitate understanding of gene expression using immunohistochemistry (IHC) and single nucleus and single cell transcriptomic.
Development and Translation of Generator-Produced PET Tracer for Myocardial Perfusion Imaging
Goal
This project translates Ga-68 Galmydar, a novel radiotracer developed by the Sharma Lab, into patients for the assessment of ischemic cardiovascular disease.
Assessment of Clonal Hematopoiesis and Its Relationship to Cardiovascular Disease in Hodgkin Lymphoma Survivors
Goal
This project plans to assess the prevalence of clonal hematopoiesis (CH) possessing somatic mutations associated with cardiovascular disease (CVD), which are detected after Hodgkin Lymphoma therapy and to assess patients with CH for the presence or absence of objective signs of CVD as measured by cardiac MRI (cMRI).
PET Myocardial Blood Flow Comparison to Coronary CTA and CT-FFR
Goal
The broad, long-term objective of this pilot study is to develop an optimal, clinically usable, noninvasive evaluation of CAD in the setting of stable angina, which provides both anatomic and functional information using PET, CCTA and CT-FFR.
PET Detection of CCR2 in Human Atherosclerosis
Goal
This project assesses the mechanism and imaging of CCR5 in carotid inflammation.
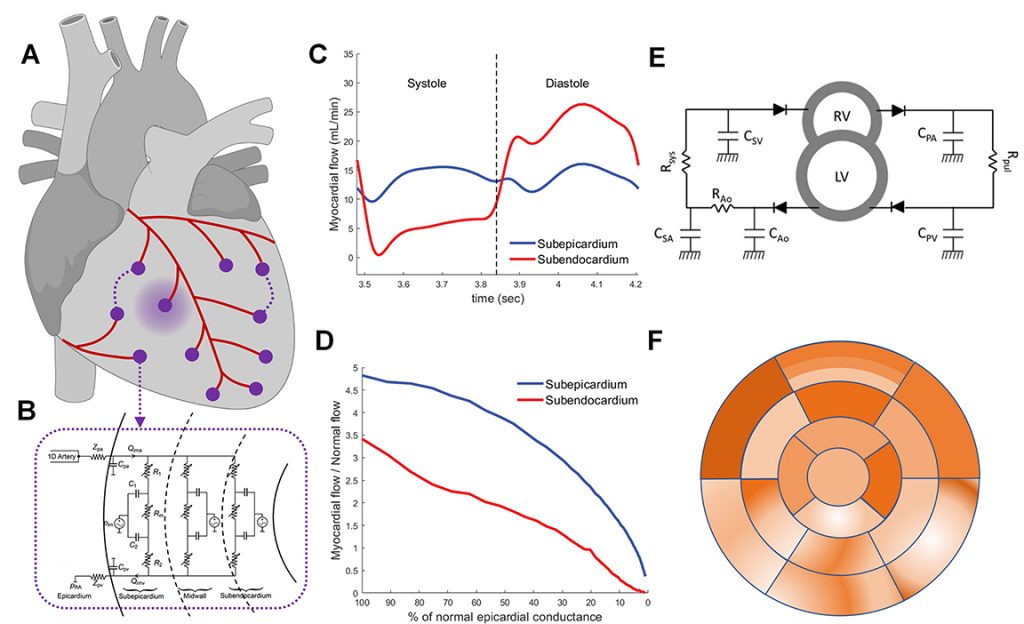
Absolute Perfusion Reserve: Theory and New Metric for Coronary Lesion Grading
Goal
The goal of our research is to develop a novel imaging-based biomarker that more accurately reflects the true physiological significance of coronary artery stenoses by accounting for patient specific properties of both the large epicardial arteries and of the microcirculation using physics and physiology based computational models informed by both anatomic and perfusion data obtained from simultaneously acquired coronary CTA and PET perfusion imaging.
Predicting Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement From Aortic Annulus Shape Models Derived From Pre-Procedural CT
Carotid Atherosclerotic Plaque Segmentation in Multi-Weighted MRI Using a Two-Stage Neural Network

Our People
The lab, led by Pamela K. Woodard, MD, consists of multidisciplinary teams with excellence in radiology, cardiology, radiochemistry, biomedical engineering and vascular biology all working together towards a shared goal.