Gordon Neuroimaging Lab
Projects
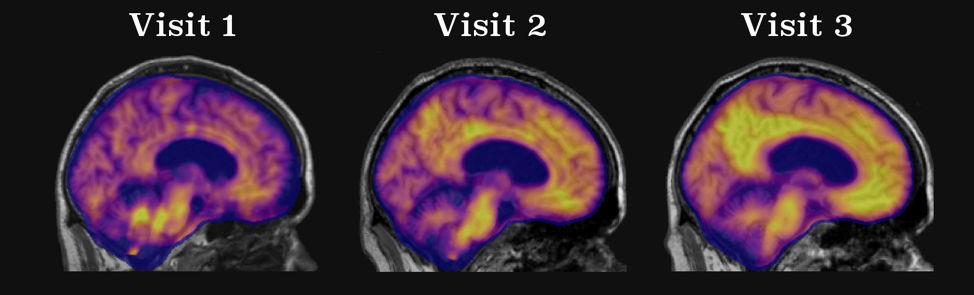
Characterizing Longitudinal Alzheimer Disease Pathology
Goal
Alzheimer Disease (AD) is an insidious process that occurs over decades. Neuroimaging provides a powerful tool to characterize the changes that occur during the preclinical phase of the disease and ultimately lead to the onset of dementia.
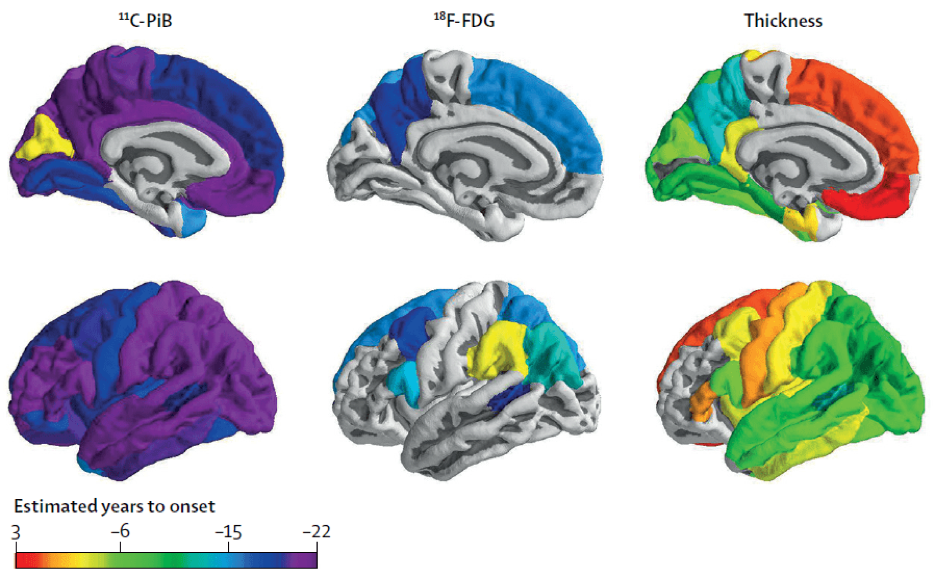
Understanding Autosomal Dominant Alzheimer Disease
Goal
Autosomal Dominant Alzheimer Disease (ADAD) is a unique early-onset form of AD caused by a mutation in the PSEN1, PSEN2, or APP genes. Individuals with these unique genes develop dementia at the same age as their parents, and as a result it is possible to accurately predict the estimated years to onset (EYO) when someone will develop dementia.
Relating Pathology to Underlying Properties of the Brain
Goal
Neurodegenerative diseases do not affect the brain ubiquitously but instead manifest in spatial patterns that mirror biological properties such as functional networks, glucose metabolism, myelination, and gene expression. A theme of research in the lab seeks to understand what properties constrain pathological expression in AD.

Our People
The lab, led by Brian Gordon, PhD, features a multidisciplinary team with a focus on integrating neuroimaging, lifestyle factors, genetics and network science to understand the biology of the brain.