NeuroPhoto Lab
Projects
High-Density Diffuse Optical Tomography
Goal
To provide a more naturalistic imaging system, but address imaging quality issues with traditional fNIRS, we developed high-density diffuse optical tomography (HD-DOT) system complete with lightweight fiber optics. HD-DOT is an emerging method that uses a dense array of overlapping light measurements to improve image quality over traditional fNIRS as validated against fMRI in adults. The naturalistic imaging environment was combined with more naturalistic imaging tasks, specifically movie viewing of traditional entertainment movies. Features across these movies, such as faces and speech, can be tracked and correlated with brain activity to visualize responses similar to standard functional imaging tasks. Using these techniques, we can map brain function in a variety of subject populations that are not amenable to fMRI, including children, subjects with cochlear implants or deep brain stimulations, or using portable systems we can image people in clinical environments


Mapping Multi-Scale Networks in Mice
Mapping spontaneous brain activity with functional magnetic resonance imaging (fMRI) and functional connectivity (FC) techniques has become a dominant neuroimaging assay for humans. FC mappings have implications for both basic neuroscience and clinical disease where fcMRI can map functional reorganization, neural plasticity and neural network integrity. As functional connectivity methods advance, a gap continues to grow between human fcMRI and the detailed genetic and molecular assays common in mouse models. In particular, the molecular and cellular underpinnings of brain-wide correlated brain activity are relatively unresolved, and an understanding of them will grow in importance as FC measures extend further into studies of disease.


Wearable High-Density Diffuse Optical Tomography
Brain imaging with MRI machines provides noninvasive access to the neural basis of development, degeneration, and disease of the brain. However, the logistics of MRI are ill-suited to many applications. Functional MRI (fMRI) is not suited for imaging subjects who cannot lie sufficiently still in MRI scanners (e.g., awake children under 5 years old) and children with disorders of voluntary movement, such as moderate to severe cerebral palsy (CP), which represents 0.2% of infants, or 10,000 annually. Many patients with implants (cochlear implants, deep brain stimulators, heart pacemakers) are also contra-indcated for MRI.
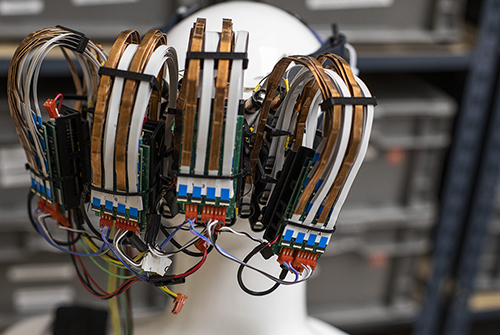
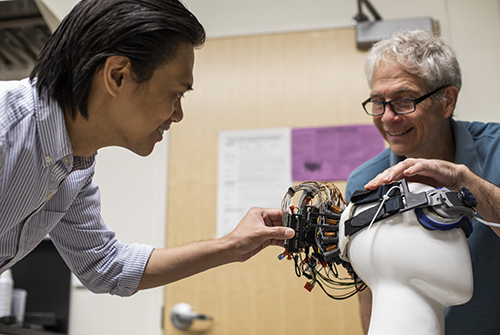
A promising potential surrogate to fMRI is high-density diffuse optical tomography (HD-DOT) a tomographic version of functional near infrared spectroscopy (fNIRS). However, despite these advances, application of HD-DOT to naturalistic studies in children has been limited by a requirement of large opto-electronic consoles and bulky fiber optics. Several wearable fiber-less fNIRS instruments are now available commercially, but traditionally these systems have had multiple deficits including either lower resolution, lower field-of-view, strong image distortions, or less signal to noise than our fiber-based HD-DOT systems. In this project we are developing a full head WHD-DOT device that matches performance of fiber-based HD-DOT for mapping brain function in typical and atypical child development.
Speckle Contrast Optical Tomography for Imaging of Cerebral Blood Flow
Goal
Real-time maps of cerebral blood flow (CBF) at the bedside are a long sought-after assay for neurocritical care. Regional CBF measures can indicate which brain regions may be becoming ischemic and are at danger for hypoxic-ischemic injury. The dominant clinical methods for mapping microvascular CBF include PET and arterial spin labeling (ASL) with MRI. However, PET and ASL-MRI provide only snapshots of CBF, not continuous monitoring, and thus miss dynamic events. Cerebral blood flow dynamics are important in many clinical scenarios, including acute stroke, traumatic brain injury and preterm birth. To monitor brain perfusion or its surrogates at the bedside clinically, several non-imaging methods are used. These include intracranial pressure (ICP), a thermal dilution method, and laser Doppler flowmetry, all of which are either very invasive or only very local measures. Unfortunately, none of these existing monitoring methods provide the desired regional map of CBF at the bedside.
Our People
The NeuroPhoto Lab, led by Joseph Culver, PhD, leverages multidisciplinary excellence in the areas of electrical engineering, biomedical engineering, computational imaging, physics and neuroscience.