“Since prostate cancer’s metastatic disease often spreads to bone, for decades conventional radionuclide bone scans were standard of care for staging and restaging men with prostate cancer,” says Barry A. Siegel, MD, professor of radiology.
Metastatic prostate cancer isn’t limited to bones, however, often traveling to lymph nodes and sometimes organs such as the liver and lungs. Conventional imaging with CT or MRI has limited accuracy for detecting these metastases. How to identify lesions throughout the body became a quest that resulted in multiple radioactive tracers being developed through the years, all with limited success.
“MIR was an early adopter of ProstaScint, the first prostate cancer imaging agent approved by the FDA in 1999, and we have been heavily engaged in research and pivotal clinical trials since then,” says Siegel, who led the division of nuclear medicine from 1973 to 2017.

That research included studies in the early 2000s using PET with 11C-acetate for the initial staging of men with newly diagnosed, high-risk prostate cancer. In November 2014, MIR received U.S. Food and Drug Administration (FDA) approval to use 11C-choline with PET/CT and PET/MRI to detect biochemical recurrence of prostate cancer. MIR also participated in the LOCATE study, which showed the clinical impact of Axumin (18F-fluciclovine), another radiopharmaceutical for PET that was approved by the FDA in 2016. Axumin is indicated for PET imaging for men with suspected prostate cancer recurrence based on elevated prostate-specific antigen (PSA) levels following prior treatment.
“These studies and others in which we participated had some success in imaging prostate cancer, but none of them produced images that gave us total confidence we were identifying all of our patients’ metastatic lesions,” says Siegel. Then, in 2020 and 2021, drugs targeting prostate-specific membrane antigen (PSMA) lesions in men with prostate cancer were approved by the FDA. Siegel and his colleagues describe these PSMA-targeted PET imaging drugs as “revolutionary” in the quality of images produced and potentially for how patients with prostate cancer will receive treatment in the future.
Understanding the Role of PSMA
All prostate cells contain low levels of PSMA, a protein that rests mainly on the cell surface. Both PSMA and another protein, prostate-specific antigen (PSA), become elevated when cancer is present. Until recently, it wasn’t known what role PSMA played in prostate cancer development; new research indicates it indirectly activates a cancer-causing pathway involving a protein kinase.
“These new PET agents bind to PSMA, which is easily accessible on the surface of prostate cancer cells,” says Tyler J. Fraum, MD, assistant professor of radiology. “The FDA approved them for use in patients with newly diagnosed high-risk prostate cancer that may be curable with surgery or other therapies, as well as in patients suspected of having recurrent prostate cancer based on elevated PSA levels.”
“We are basically reinventing how we stage prostate cancer.”
MIR played a significant role in trials evaluating Pylarify (18F-piflufolastat) for FDA approval, serving as a lead enroller of patients in its OSPREY and CONDOR clinical trials. Siegel was a senior author on papers summarizing the clinical trials’ findings.
“The studies showed that these PSMA-targeted drugs identify far more prostate cancer lesions than any other tracers used in the past,” says Siegel. “The result is, we are basically reinventing how we stage prostate cancer, both initially and at the time of recurrence, because we are finding disease we had no knowledge of previously.”
This means patients may be identified as having a higher stage of cancer than initially diagnosed — for instance, stage 1 may now be classified as stage 2, stage 3 may be stage 4. “Additional study needs to be done to determine the impact of this stage migration on patient outcomes, as well as to decide whether prostate cancer treatments need to be changed to address the new information these improved scans are providing,” says Siegel.
The Advent of Theranostics
The new drugs also make possible the integration of therapy and diagnostics in nuclear medicine, according to Farrokh Dehdashti, MD, the Drs. Barry A. and Marilyn J. Siegel Professor of Radiology and senior vice chair and division director of nuclear medicine. Called theranostics, this combination focuses on patient-centered care that provides treatment based on whether a patient’s cancer demonstrates affinity for the diagnostic form of a drug.
“Theranostics basically means we treat what we see,” she says. “The first step is tagging a PSMA-targeted drug with a diagnostic agent to identify metastatic prostate cancer lesions. If we determine the patient will benefit from treatment, the second step is tagging the same drug with a therapeutic radioactive agent that delivers a dose of radiation specifically to the area where lesions are overexpressing PSMA. Since the therapy is targeted specifically to the PSMA on prostate cancer cells, surrounding tissues are less likely to be affected by the therapy.”
PET/MRI: A Combined Advantage
PSMA-targeted PET imaging drugs will play a role in a project under development by Fraum as well. With training in both diagnostic radiology magnetic resonance imaging (MRI) and nuclear medicine PET imaging, Fraum interprets both PSMA PET scans and prostate MRIs conducted in abdominal imaging. He is using this dual expertise to expand MIR’s capabilities in offering the hybrid imaging modality of PET/MRI scans.
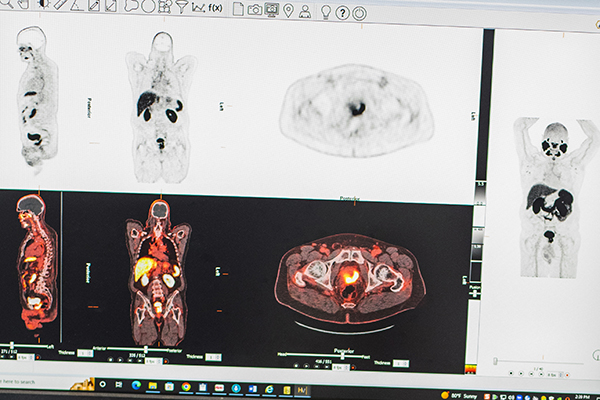
“Patients with newly diagnosed or biochemically recurrent prostate cancer often undergo both MRI and PET scans,” says Fraum. “The MRI identifies the local extent of disease, whereas the PSMA PET is most useful for identifying regional nodal or distant sites of cancer spread.”
PET/MRI combines both scans into a single examination, a one-stop shop for patients. It has significant advantages from an interpretive perspective too.
“PET/MRI enables us to see more precisely the correlation between findings on those two separate examinations in a way that’s not always possible if they are conducted at different times,” explains Fraum. “Structures in the pelvis can shift — for example, the bladder filling and emptying — and it’s not always clear when looking at the two scans performed separately whether a finding of concern on one study corresponds with the same finding of concern on the other. When PET and MRI are done at the same time, we have near-perfect co-registration of those images.”
The Nuclear Medicine Evolution Continues
Even with significant advancements such as PSMA-targeted PET imaging drugs, investigators continue to explore ways to improve the technology.
“There are new PSMA tracers in the development pipeline that may perform even better than the tracers currently available to us,” says Fraum. “An example is a PSMA tracer that doesn’t undergo as much urinary excretion. Tracer in the urinary bladder can interfere with the detection of prostate cancer in the pelvis.”
Although MIR’s Division of Nuclear Medicine historically has concentrated on the diagnostic aspect of theranostics, Dehdashti notes that upcoming clinical trials will focus on the division administering the therapeutic dose as well. She adds, “And we will continue participating in nuclear medicine imaging clinical trials that we feel best serve our patient population.”
Siegel sees the division’s decades-long history of research into prostate cancer imaging as an evolution. “We moved from tests that weren’t as useful as we hoped they would be to the tests available today that are changing the way we diagnose and treat the disease. It’s gratifying to be able to provide information that significantly impacts how patients are managed.”
Published in Focal Spot Fall/Winter 2022 Issue